Immature Granulocytes in PBFs
1. Myeloblast
Background Information of Myeloblast
The myeloblast is the first recognizable unipotent stem cell (most immature), which will differentiate into one of the effectors of the Granulocyte series and this process is called granulopoiesis. The granulopoiesis consists of 5 stages, with the next differentiation sequence being pro-myelocyte. This cell have the ability to differentiate into one of the three different precursor cells, the neutrophilic, basophilic or eosinophilic myelocyte. The myeloblast is exclusively found in the bone marrow in normal circumstances and will not be found on PBFs. Presence of this cell type will indicate severe hematological disorders.
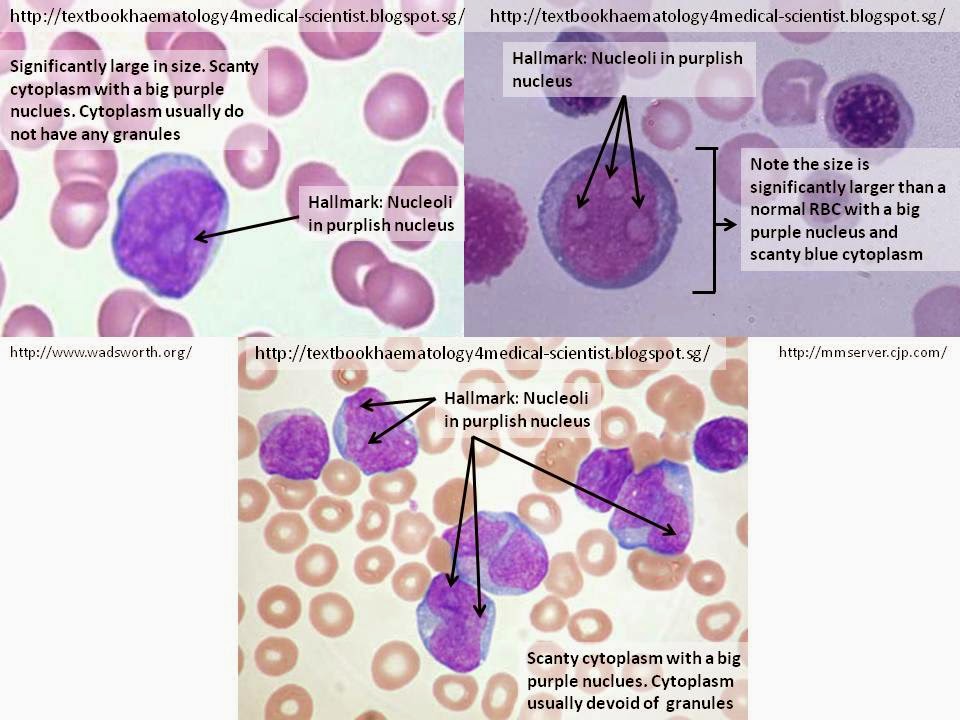
Cellular Description
The hallmark of the myeloblast is the presence of 2-5 nucleoli that are brighter or lightly stain compared to their surrounding nucleus. The cell commonly appear as a significantly large round shape (maybe irregular at times) compared to the RBCs with relatively scanty blue cytoplasm and a large irregular purple nucleus. The cytoplasm is usually devoid of granules and the nucleus is composed of very fine non-aggregated chromatin.
2. Promyelocyte
Background Information of Promyelocyte
The Promyelocyte is the second differentiation sequence after differentiation from myeloblast in the granulopoiesis after differentiation.
Cellular Description
The hallmark of the Promyelocyte is the presence of coarse azurophilic (purplish) granules in the cytoplasm and may overlie the nucleus. The size of promyelocyte is usually larger than myeloblast with a reduced nuclear:cytoplasm ratio, meaning that the cytoplasm is more plentiful with a relatively smaller round/oval shaped nucleus compared to myeloblast. The cytoplasm staining varies between pinkish to bluish but the nucleus remain dark purple and the nucleus is usually skewed to a side of the cell (ie: not in the middle). Occasionally, nucleoli can still be present in the nucleus.
3. Myelocyte
Background Information of Myelocyte
The Myelocyte is the third differentiation sequence after differentiation from Promyelocyte in the granulopoiesis after differentiation.
Cellular Description
The hallmark of the Myelocytes is the presence of fine lightly stained granules in the pinkish plentiful cytoplasm with a much relatively smaller round nucleus than both Promyelocyte and Myeloblast. The chromatin in the nucleus is stained more darkly in purple as it is more condensed and should not have any nucleoli. Furthermore, the nucleus is usually lying eccentrically. Myelocytes are significantly smaller than promyelocytes and have a 1:1 ratio of nucleus:cytoplasm.
4. Metamyelocyte
Background Information of Metamyelocyte
The Metamyelocyte is the fourth differentiation sequence after differentiation from Myelocyte in the granulopoiesis after differentiation. This is the last stage where these cells are considered immature.
Cellular Description
The hallmark of the Metamyelocyte is the presence of a large kidney-shaped or broad V shape nucleus in pinkish plentiful cytoplasm at the ratio of 1:1, cytoplasm:nucleus. There will not be any nucleoli in the nucleus nor there will be coarse granules in the cytoplasm. There will be fine lightly stained granules. It is the smallest in size in the immature granulocyte series when compared to myeloblast, promyelocyte and myelocyte, but almost similar in size with segmented/band neutrophils.
Discrepancy
Metamyelocytes are commonly mistaken as band neutrophils and vice-versa. It is a good practice to differentiate metamyelocytes from band neutrophils from the shape of nucleus. The nucleus of a band neutrophils has a much deeper band while a metamyelocyte has a shallow band.